
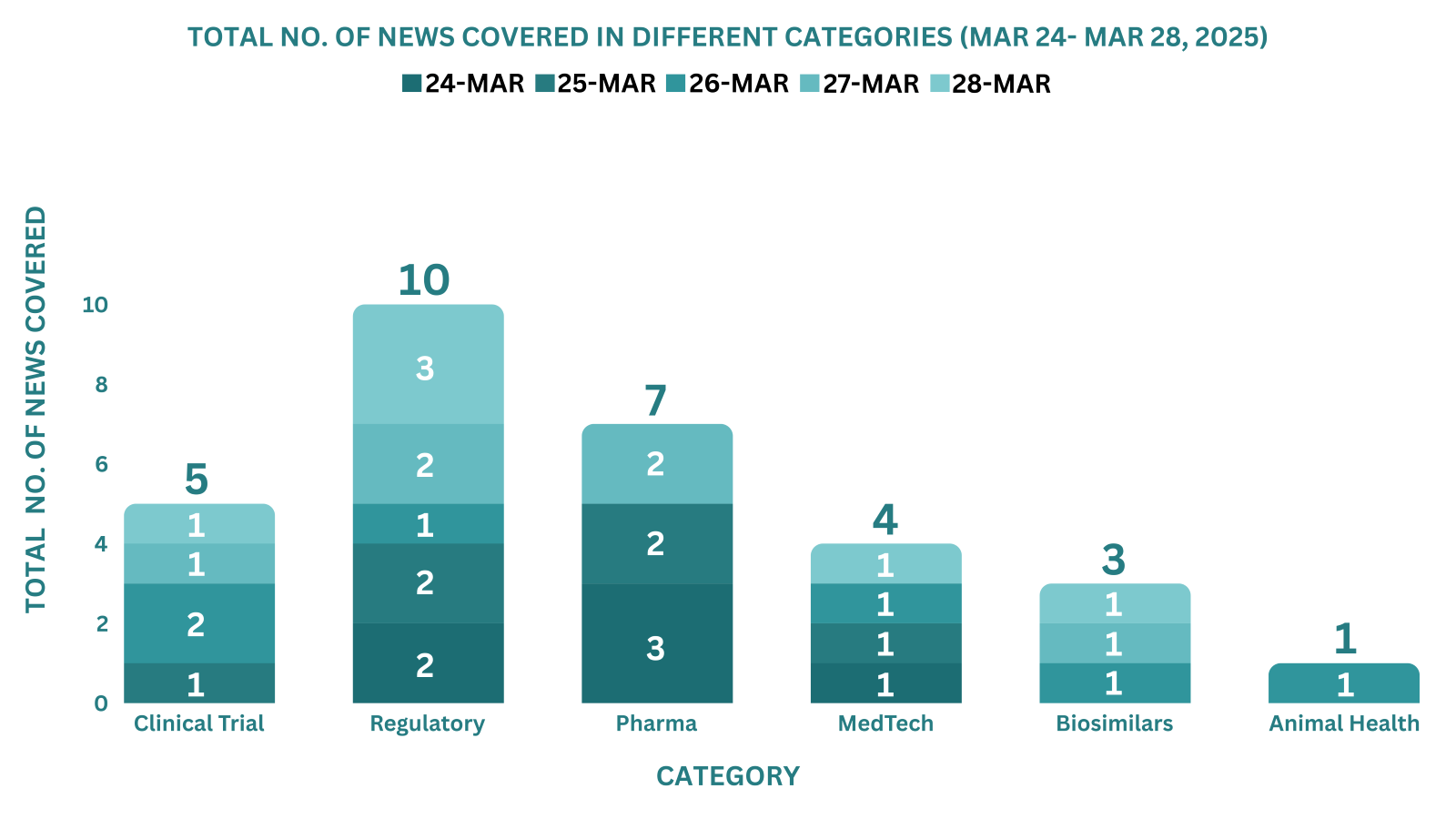
PharmaShots Weekly Snapshots (March 24, 2025 – March 28, 2025)
This week PharmaShots’ news was all about the updates on Clinical Trials, Regulatory, Pharma, MedTech, Biosimilars & Animal Health. Check out our full report below:
AstraZeneca to Highlight Tagrisso’s Clinical Data for NSCLC at ELCC 2025
Read More: AstraZeneca
Accent Therapeutics to Highlight Preclinical Data of ATX-559 and ATX-295 at AACR 2025
Read More: Accent Therapeutics
Axsome Therapeutics Reports P-III (FOCUS) Trial Data of Solriamfetol for Attention Deficit Hyperactivity Disorder (ADHD)
Read More: Axsome Therapeutics
Johnson & Johnson Reports P-III (MARIPOSA) Trial Data of Rybrevant + Lazcluze for EGFRm NSCLC
Read More: J&J
Merck Presents P-III (3475A-D77) Trial Data of SC Keytruda for NSCLC at ELCC 2025
Read More: Merck

China’s NMPA Approves Junshi Biosciences’ Toripalimab + Bevacizumab for Advanced Hepatocellular Carcinoma
Read More: Junshi Biosciences
GSK Reports the EMA’s Acceptance of Nucala (Mepolizumab) as an Add-on Treatment for COPD
Read More: GSK
Sanofi Reports the US FDA’s Acceptance & Priority Review of Tolebrutinib for Non-Relapsing Secondary Progressive Multiple Sclerosis
Read More: Sanofi
SineuGene Therapeutics Reports the US FDA’s IND Clearance of SNUG01 for Amyotrophic Lateral Sclerosis
Read More: SineuGene Therapeutics
GSK Reports the US FDA’s Approval of Blujepa (Gepotidacin) to Treat Uncomplicated Urinary Tract Infections (uUTIs)
Read More: GSK
Exelixis Reports the US FDA’s Approval of Cabometyx (Cabozantinib) to Treat Advanced Neuroendocrine Tumors (NETs)
Read More: Exelixis
Merck Secures the EC’s Approval for Capvaxive (Pneumococcal 21-valent Conjugate Vaccine) for Invasive Pneumococcal Disease (IPD)
Read More: Merck
BridgeBio Receives the MHLW’s Approval for Beyonttra (Acoramidis) for Transthyretin-Mediated Amyloidosis Cardiomyopathy (ATTR-CM)
Read More: BridgeBio
Sanofi and Regeneron’s Dupixent Receives the MHLW’s Approval to Treat Chronic Obstructive Pulmonary Disease (COPD)
Read More: Sanofi and Regeneron

AstraZeneca Collaborates with Syneron Bio to Develop Macrocyclic Peptides for Chronic Diseases
Read More: AstraZeneca & Syneron
Harbour BioMed & AstraZeneca Partner to Develop Multi-Specific Antibodies across Various Therapeutic Areas
Read More: Harbour BioMed & AstraZeneca
The United Laboratories (TUL) and Novo Nordisk Enter exclusive license agreement for UBT251
Read More: TUL and Novo Nordisk
Alumis and Kaken Pharmaceutical Enter Collaboration & Licencing Deal for ESK-001’s Development & Commercialization in Japan
Read More: Alumis and Kaken Pharmaceutical
Merck Partners with Jiangsu Hengrui Pharmaceuticals for HRS-5346, Expanding its Cardio-Metabolic Portfolio
Read More: Merck and Jiangsu Hengrui Pharmaceuticals
Bayer Enters Global License Agreement with Suzhou Puhe BioPharma for its PRMT5 Inhibitor
Read More: Bayer
Ionis Partners with Sobi for Olezarsen to Treat Familial Chylomicronemia Syndrome (FCS) & Severely Elevated Triglycerides
Read More: Ionis and Sobi

Newronika's AlphaDBS Receives European CE Mark for Adaptive Deep Brain Stimulation in Parkinson's Disease
Read More: Newronika
Avatar Medical & FX Shoulder Solutions Reports the US FDA’s 510(k) Clearance of brAIn Shoulder Positioning System (SPS) for Arthroscopic Surgeries
Read More: Avatar Medical & FX Shoulder Solutions
Huntleigh Healthcare Reports the US FDA’s 510(k) Clearance of Dawes-Redman CTG Analysis to Improve Non-Stress Test Interpretation in Fetuses
Read More: Huntleigh Healthcare
Abbott Secures European CE Mark for Volt PFA System to Treat Atrial Fibrillation
Read More: Abbott

Zoetis Reports Clinical Data of Librela for Canine Osteoarthritis Pain
Read More: Zoetis

Alvotech, Kashiv Biosciences and Advanz Pharma Report the MHRA’s MAA Acceptance of AVT23 (Biosimilar, Xolair)
Read More: Alvotech, Kashiv Biosciences and Advanz Pharma
The US FDA Approves Fresenius’ Conexxence and Bomyntra (Biosimilars, Prolia & Xgeva)
Read More: Fresenius
Related Post: Pharmashots Weekly Snapshots (March 17, 2025 - March 25, 2025)
Tags

Ridhi is an avid secondary researcher who follows trends in the biopharmaceutical and healthcare sectors to curate engaging content for the global audience. She works as a news editor at PharmaShots and loves to read books and explore new destinations.














